Introduction
In dairy industry, the use of enzymes, particularly exogenous enzymes are not fully exploited and limited to a few major and some minor applications. Enzymes play important role in the preparation of certain dairy products like cheeses, yoghurt etc., by improving texture, flavor and bringing about desirable changes in the product. Lipolytic and proteolytic enzymes can accelerate the production of flavor compounds. Successful use of preparations containing these enzymes is complicated by the need to attain a satisfactory balance among the various enzymes involved in the cheese ripening process.
An immobilized enzyme is an enzyme that is physically attached to a water-insoluble matrix, supporting material, or carrier. The immobilized enzyme is unable to move due to its linkage to the support or matrix, and its phase difference from substrates and products. Carriers, matrixes, or supporting materials frequently used are: ceramics, spongy glass, cellulose, sand, synthesized polymers, charcoal, metal oxides, stainless steel and polymeric gel. Today, in many cases immobilized enzymes have revealed highly efficient for commercial uses. They offer many advantages over enzymes in solution, including economic convenience, higher stability, and the possibility to be easily removed from the reaction mixture leading to pure product isolation. An immobilized enzyme is, therefore, attached to an inert, organic, or inorganic or insoluble material, such as calcium alginate or silica. Furthermore, the attachment of an enzyme to a solid support can increase its resistance to various environmental changes such as pHor temperature.
Immobilization of Enzymes
It is always cost-effective to use the enzymes more than once. Most of the enzymes are in solution with the reactants and/or products and hence it is difficult to separate them after completion of chemical reaction. In order to reuse the enzymes again after separation from the products during chemical reaction, it is necessary to employ techniques that are helpful in attaching the enzyme to the reactors. This idea has led to the employment of immobilization techniques for enzymes. The concept of enzyme immobilization was first evolved, when difficulties were experienced during the use of crude enzyme preparations in the production of wine, cheese or in tanning. The phenomenon of immobilization of enzyme on a support, was first reported by J.M. Nelson and E.G. Griffin in 1916. They reported the adsorption (immobilization) of invertase on charcoal/alumina without loss of activity. However, the technique of enzyme immobilization could be established only after a lapse of about 40 years, in 1954- 1961 when many researchers developed relevant procedures and the equipment’s.
In simple terms “immobilized” means unable to move or stationary. An immobilized enzyme is an enzyme which is attached to an inert, insoluble material such as calcium alginate over which a substrate is passed and converted to product. This technique has revolutionized the prospects of enzyme application in industry.
Immobilization is defined as the imprisonment of a biocatalyst in a distinct phase that allows exchange with, but is separated from, bulk phase in which substrate, effectors, inhibitor molecules are dispersed and monitored.
Immobilized enzyme is physically entrapped or covalently bonded by chemical means to an inert and usually insoluble matrix, where it can act upon its natural substrate. The matrix is usually a high molecular weight polymer such as polyacrylamide, carrageenan, chitin, cellulose, starch, glass beads, etc.
Advantages:
- Immobilization allows separation of enzymes from the products after completion of chemical reaction and thus can be reused or recycled.
- Immobilized enzymes have ability to bind to a matrix, by which it typically possess greater resistance to change in pH and temperature and have operational stability than the soluble form of the enzyme.
- Reaction mixture or products specifically contain only solvent and reaction products and so more or less do not require complex purification as the processed product is not contaminated with the enzyme.
- Immobilization improves the efficacy and efficiency of an enzyme.
- Certain manipulations of chemical reactions are better possible with immobilized enzymes
Methods of immobilization
The Traditional Methods of Enzyme Immobilization can be classified as follows:-
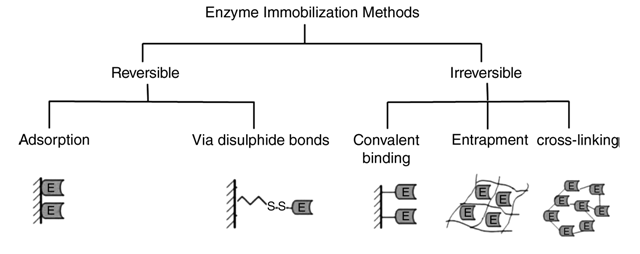
1.Carrier binding
In this method, the enzymes are bound to water in-soluble carrier molecules. Based on the technique of binding, this is further divided into :-
(a)Physical adsorption:-This is the immobilization of an enzyme on the surface of water-insoluble carriers.
(b)Ionic binding: – This process involves ionic binding of the enzyme to water-insoluble carriers containing ion-exchange residues.
2.Covalent binding
This is based on the binding of enzymes and water-insoluble carriers by the formation of covalent bonds between the enzyme and the support matrix. e.g. Glutaraldehyde
3.Cross linking
This is the process of intermolecular cross-linking of enzymes by bi-functional or multi- functional reagents.
4.Entrapment
Here enzyme is trapped in insoluble beads or microspheres i.e. incorporating enzymes into the lattices of a semi-permeable gel or enclosing the enzymes in a semi-permeable polymer membrane.
Whole Cell Immobilization
The term “immobilized cells” refers to keeping the cell in one place. Generally, in a reaction environment, cells are floating around in nutrient liquid whereas in cell immobilization, the cells are trapped or stuck to a sticky surface while nutrient flows over them. This method is more appropriate and useful when there is a need for using or when a series of enzymes are required in the process.
Whole cell immobilization was defined as “the physical confinement or localization of intact cells to a certain region of a space with preservation of some desired catalytic activity”, and is a process that often mimics what occurs naturally when cells grow on surfaces or within natural structures.
Advantages of whole-cell immobilization
- Enzyme isolation and purification steps are not required
- Show the higher stability in the catalytic power and enzyme activity,
- Have multivariate enzyme applications, eliminating the need for immobilization of multiple enzymes
- Intracellular enzymes need not be extracted prior to the reaction; they may be used directly
- The end products can be recovered in a simple manner.
- The technique is cost effective.
Disadvantages
- Low productivity
- Lower resistance
- Limitation of mass transfer
- Problems with degradation of product
- Byproducts are formed due to lysis of cells or toxic metabolites
The release of cells growing in the peripheral layer of highly colonized gel beads can be used to efficiently produce biomass in the bulk liquid medium. This cell release activity can be used for producing single or mixed strain cultures and to continuously inoculate food liquids to process fermented foods such as fermented milk products, bulk lactic starter cultures, mass Probiotic cultures and pre-fermentation of milk. The immobilized cell technology can be used for production of different metabolites and functional ingredients from LAB using this low value whey permeate containing high lactose and mineral contents, used as a culture medium for the production of lactic starter cultures or metabolites .Lactic acid production, Exo polysaccharide production and Bacteriocin production are few more applications. The other food industries like in beer and wine making are the best examples of industrial exploitation of immobilized cell technology

Shubha Singh*1 and Neha Thakur1
1Department of Livestock Products Technology, Lala Lajpat Rai University of Veterinary and Animal Sciences, Hisar
*Correspondence: drshubha.singh06@gmail.com