Bacillus-based probiotic products containing bacterial spores seem to be particularly well-suited for use in broiler feeds. In the spore form, they are metabolically dormant and resilient to environmental stresses, including pelleting. There have been years of debate on the mode of action of probiotics in chickens and more broadly in poultry. A cornerstone of the debate centers on the ability of spores to germinate and become viable organisms in the intestine due to the rapid transit time in the poultry gastrointestinal tract (GIT). These aspects were clarified in 2008 by Cartman et al. The research showed that orally-administered Bacillus subtilis spores germinate in the chicken’s GIT (Cartman et al. 2008). Continuous administration of an effective Bacillus subtilis probiotic is advisable to achieve persistent benefits (Latorre et al. 2014). Another point of discussion has focused on whether spore-forming Bacillus spp. are transient organisms in the gut or if they could attach somehow to the intestinal epithelium. This led to two schools of opinion in the scientific community.
To answer this question, the Innovation Department of Chr. Hansen, A/S performed a special fluorescence experiment in collaboration with the Department of Animal Nutrition, of the Kielanowski Institute of Animal Physiology and Nutrition, Polish Academy of Sciences, Jabłonna, Poland.
A Recent Study Answers the Debate
The most recent study was conducted to investigate the effects of commercially available spores of Chr. Hansen Bacillus subtilis spore-based probiotic in diets at 1.6 × 106 CFU/gram of feed. Performance parameters and microbiota activity in the broilers were assessed. Fluorescence in situ hybridization (FISH) was performed to investigate the spatial organization and the formation of Bacillus subtilis biofilms in intestinal samples from various GIT locations in 6 broiler chickens. Tissue sections from each chicken were analyzed in duplicate and visualized by fluorescence microscopy with a 40x objective.
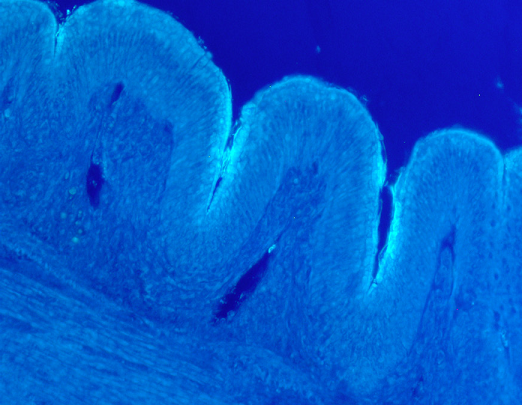
Do Bacillus Subtilis colonize the gut or are they transient? Both.
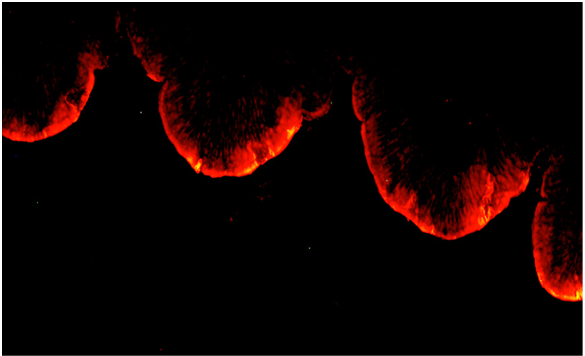
Indeed, the first picture (Figure 1), describes very well how B. subtilis colonize the intestinal epithelium in the intestine. We can see very clearly the red fluorescence on the surface of the villi of the intestine.
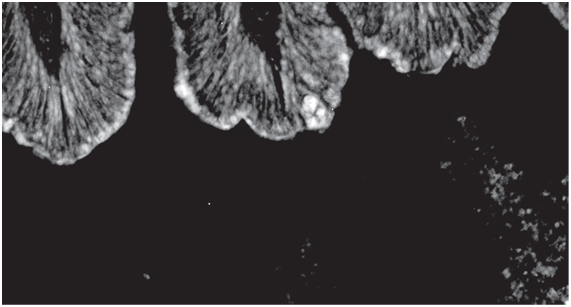
In the second picture (Figure 2) a different fluorescence is observed. Some luminescence inside the lumen of the intestine is seen, which clearly shows that transient Bacillus are in the intestine. The bacteria are alive and multiplying
into the lumen of the gut content.
Bacillus are at the right place to act!
This picture is interesting, it clearly depicts that Bacillus are able to colonize the surface of the villi. This is an excellent place to be in the intestine. The top of the villi represents one of the most sensitive sites of the epithelium. This is the place where a lot of nutrients are absorbed due to the full development of the microvilli. This is also the place where most of the pathogens are acting to destroy the mucosae (C. perfringens, E.coli, Salmonella).
Several advantages can be deduced by coating the epithelium surface.
Presence on the villi equates to improved total intestinal surface
Many different publications have documented that the use of Chr. Hansen probiotics result in an increase in the length of villi and an increase of the very well-known Villi length/Crypt Ratio (Boroojeni F.J. et al, 2018). This indicator is typical of enhanced intestinal functionality. It is easily understandable that when the surface of absorption is increased, the efficiency of the nutrient absorption through the epithelium correspondingly improves. By coating the surface of the villi, Bacillus are able to protect the integrity of the villi and microvilli and subsequently prolong the life cycle of the cells (typically about 4 to 5 days) before their expulsion in the lumen of the intestine.
Presence on the villi results in bacteriocin effectiveness
Some Bacillus spp. are specifically strong in bacteriocin production. A bacteria can be defined as an inhibitory peptide against unfavorable bacteria.
For instance, those peptides are known to inhibit the growth of C. perfringens but also more recently of E. coli and Salmonella.
Presence on the villi improve metabolite and enzyme production
Bacillus spp. can produce and release multiple active enzymes in the intestinal tract. The principal objective of these enzymes is to digest the undigestible part of the feed which may be in the micro-environment surrounding the bacilli colonies. Once these enzymes are released, they continue to act and cut the complex insoluble or indigestible fraction of feed into smaller pieces which are then readily absorbable by the microvilli. The presence of the Bacillus on the surface of the epithelium makes these enzymes act exactly as necessary for the bird’s absorption. On top of this, a recent paper demonstrated the capacity of increased butyrate production in the intestine (Konieczka P et al., 2018).
Conclusions
This most recent research helps to further understand an important part of the mode of action of effective Bacillus probiotics.
Therefore, Bacillus probiotics:
• Can germinate in the gut and become an active part of the bacteria microbiome in poultry.
• Can be transient, live organisms in the flow of the intestinal content.
• Can colonize the surface of the intestinal villi resulting in three major benefits for the bird’s intestine:
• Protection of the surface of the villi, thus prolonging and protecting this very important part of total nutrient absorption
• Creating the right place for bacteriocin production, resulting in an unfavorable micro-environment for pathogens such as C. perfringens, Salmonella, and E. coli,
• Releasing enzymes and butyrate locally, close to the brush surface epithelium. This enables the digestion of the indigestible part of the feed and improves the digestibility of key elements of the feed. Over years of controversial debate, the answer from science is showing us again that we are just scratching the surface of the probiotic potential in poultry production. This study confirms there is a bright future for this technology, and it is supported by science-based evidence.
Author:
Christophe Bostvironnois, Global Product Manager
Jean-Christophe Bodin, Technical Product Manager
John Schleifer, Technical Product Manager North America
Dorthe Sandvang, Innovation Department Manager









