1 Introduction
1 Gut health can be defined as the ability of the gut to perform normal physiological functions and
2 to maintain homeostasis and thus supporting its ability to withstand infections and non-infectious
3 effective digestion and absorption of food, a stable gut microbial population, structure and function
4 of the gut barrier, and effective function of the immune system, all of which play a critical role in
5 gut physiology, the productivity of the animal and its well-being. Over the past two decades, this
6 topic has gained even more interest in poultry production due to increasing demands for economic
7 efficiency, animal welfare, food safety, reduction in environmental impacts, and a ban on or
8 avoidance of antibiotic growth promoters (AGPs) use (Morgan, 2017). The exogenous enzymes
9 are capable of reducing the variability in feed ingredients and enhance the feed digestibility
10 availing more nutrients for absorption and thus reduce digesta viscosity. Recently the enzyme
11 culture has gained immense value in poultry industry and this has led to development of more
12 potential enzyme combinations to target specific substrates in feed and also complement to
13 endogenous enzymes. In this article, the role of exogenous enzymes emphasizing particularly
14 carbohydrase on gut health in poultry is mainly depicted.
15 Factors responsible for gut health impairment
16 The common aspects affecting broiler gut health are stress, exogenous infection, diet and water
17 etc. Recently, with the advancement of exogenous enzyme study, more studies have been
18 conducted on the impairment factors of the intestinal health of broilers focusing on phytic acid,
19 non-starch polysaccharides (NSPs).
20 Non-starch polysaccharides, together with resistant starch and lignin called the dietary fiber, are
21 found in plants especially in the endospermic cell wall of multiple kinds of seeds (Lovegrove et
22 al., 2017). NSPs can be divided into soluble and insoluble fractions. Soluble NSPs when fed in
23 bulk amount increase the viscosity of intestinal contents by making viscous gels which decrease
24 the rate of diffusion of endogenous digestive enzymes and substrates with hampered interaction at
25 the mucosal surface (Raza et al., 2019). This increased viscosity also induces thickening of the
26 mucous layer in the intestine (Hedemann et al., 2009) hampering the digestion and absorption of
27 nutrients in the intestinal tract. It has been estimated that 400-450 kcal of digestible energy per kg
28 of feed remains undigested due to the NSP contents present in corn-soybean meal diets (Cowieson,
29 2010). On the other hand, insoluble NSP present in the cell wall entrap starch, protein and other
30 nutrients inside called “cage effect” and hinder the access of endogenous enzymes to digestible
31 nutrients (Bedford and Partridge, 2010).
32 Carbohydrases
33 The major barriers of the intestinal tract are mucus layer and tight junctions (TJ) of the epithelium
34 as illustrated in Fig. 1b. Intestinal morphology (villus height, crypt depth and epithelial turnover
35 rate) changes in response to exogenous agents, for example, presence or absence of food and
36 pathological conditions (Gomide Junior et al., 2004). Deeper crypts indicate faster tissue turnover
37 as they contain stem cells and considered villus factories (Awad et al., 2009). Intestinal
38 mucins/mucous are high molecular weight glycoproteins secreted by goblet cells. In chickens,
39 mucin-2 is observed to be extensively expressed in goblet cells of colon and small intestine
40 (Smirnov et al., 2005). NSP have been shown to increase mucin secretion (Tanabe et al., 2006) as
41 illustrated in Fig. 1c. Therefore, NSP lessen the digestion and absorption of nutrients through its
42 physicochemical effect in the intestinal tract. As a result of high fiber diets, undigested/unabsorbed
43 nutrients change in microbial populations in the gut (Bird et al., 2007; Choct et al., 1999;
44 Mathlouthi et al., 2002). Langhout (2000) observed that dietary NSP considerably decrease
45 beneficial bacteria while increases intestinal populations of pathogenic bacteria. Exogenous
46 enzymes improve digestion in the small intestine and reduce the amount of substrate availability
47 for putrefactive and starch utilizing bacteria in the large intestine. Also enzymes help in the disease
48 prevention by to reducing digesta viscosity (Pluske et al., 1997) as illustrated in Fig. 1d. Xylanase
49 and glucanase supplementation in barley, wheat, oats, and rye based diets significantly raised
50 caecal butyrate and acetate concentrations, but such effect was absent in hull-less varieties of
51 barley and oats (Jozefiak et al., 2006). Degradation and solubilisation of NSP by feed enzyme
52 increases available substrates (oligosaccharides or mono-saccharides) for microbial fermentation
53 in the cecum (Cadogan & Choct, 2015), and results in decreased VFA/SCFA production in the
54 ileum suggesting decreased fermentation whereas caecal fermentation markedly increased. The
55 increment in caecal fermentation resulted an influx of xylo-oligosaccharides (XOS) which
56 produces VFA/SCFA and energy from indigestible substrates and often leads to a healthier
57 microflora (lactic acid bacteria, LAB) (Jia et al., 2009). Therefore, the NSP fraction supplemented
58 with EFE represents another potential energy reservoir to increase the performance of broilers if
59 rendered fermentable.
60 Xylanase is a non-starch polysaccharide (NSP) degrading enzyme which cleaves the internal β-
61 xylosidic glycosidic linakges of linear xylan chains to xylo-oligosaccharides (Jompengmuengbout
62 et al. 2009), resulting in a mixture of arabinose-substituted xylo-oligosaccharides (arabinoxylan-
63 oligosaccharides, AXOS) and non-substituted xylo-oligosaccharides. As an energy source,
64 probiotics (beneficial bacteria like Lactococcus, Lactobacillus and bifidobacterium) have
65 significantly higher XOS utilization efficiency than pathogenic bacteria, especially
66 bifidobacterium which is comparable to glucose in XOS utilization efficiency. Secondly, SCFAs
67 are mainly produced by beneficial microorganism and, thirdly, SCFAs can improve pH values in
68 gut and contribute to a suitable environment for beneficial microbes which prefer acidic
69 environment, also serves as an energy source for intestinal epithelial cells. So, the XOS can be
70 utilised more efficiently and it also potentiates the activity of endogenous digestive enzyme and
71 reduces the availability of indigestible substrates for microbial growth and as a result digesta
72 viscosity is decreased leading to reduced microbial populations in the upper tract and there is
73 reduced loss of endogenous amino acids through modifications to pancreatic amylase and mucin
74 secretion (Cowieson and Bedford 2009). The prebiotic effects of XOS also include optimisation
75 of colon function, alter the amount and ratio of SCFAs and thus providing more energy,
76 augmenting mineral absorption, immune stimulation and increased ileal villus length (Kiarie et al.
77 2014). Also, researches have shown that xylanase supplementation can improve chicken immunity
78 (Gao et al., 2007), reduce the detrimental effect of Salmonella typhimurium infection (Vandeplas
79 et al., 2009; Amerah et al., 2012), or alleviate the intestinal mucosal barrier impairment of broiler
80 chickens challenged by Clostridium perfringens (Liu et al., 2012).
81 Soybean meal (SBM) is a primary source of vegetable protein that contains 3% soluble NSP and
82 16% insoluble NSP (Irish and Balnave, 1993), consisting mainly of mannans and galactomannans
83 (Slominski, 2011). Beta-mannan (β-mannan), also referred to as beta-galactomannan (βGAL), is
84 a polysaccharide that has repeating units of mannose containing galactose and/or glucose (Hsiao
85 et al., 2006). Although βGAL content of SBM is in relatively low concentrations, it is a concern
86 for nutritionists due to the presence of anti-nutritive properties (Arsenault et al., 2017). ß-mannan
87 has a molecular structure similar to some pathogens, which may trigger immune stimulation.
88 Acemannan (ß-1,4-acetylated mannan) induced the activation of macrophages via increasing the
89 nitric oxide synthase level at transcription level as reported by Ramamoorthy et al. (1996). Karaca
90 et al. (1995) reported that nitric oxide acts as a cytostatic effector in the removal of viral replication
91 and is proposed to be toxic for tumor cells (Karupiah et al., 1993). The response of this complex
92 to ß-mannan containing compounds could lead to losses in dietary energy utilization.
93 Supplementation of ß-mannanase improved the utilization of dietary energy in corn-soya diet in
94 broiler chickens (Li et al., 2010) as well as layers (Wu et al., 2005) (Saeed et al., 2019).
95 Conclusion
96 Diets with higher soluble NSP increase intestinal viscosity and reduce nutrient digestibility and
97 have negative impact on the bird’s health and performance. Exogenous NSPase enables digestion
98 process of a broad range of dietary fibers lowering intestinal viscosity and competition between
99 host and microbiota for SCFA in the small intestine and improve digestibility of nutrients.
100 Therefore there is an overall improvement in intestinal health and load of pathogenic microbes.
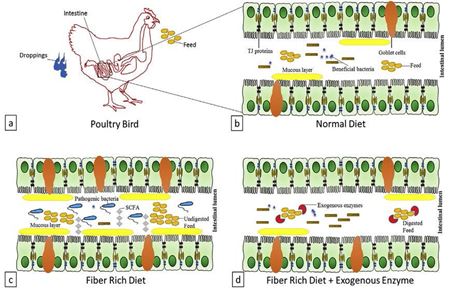
101 Fig 1. Fibers, EFE and intestinal health. (a) poultry bird, (b) intestinal lumen presenting normal goblet cells, TJ proteins, mucous layer, feed,
102 beneficial cells and enterocytes, (c) intestinal lumen presenting highly viscous environment with increased mucous, undigested feed, competition
103 of host and microbiota for SCFA in small intestine, (d) intestinal lumen presenting carbohydrases, normal mucous, beneficial bacteria and digested
104 feed. (Adapted from Raza et al., 2019)
- Dr. Preeti Puspa Mohanty
- Technical Marketing Manager, CJ Bio









