

Environmentally conscious consumers place a premium on global sustainability, animal welfare, and obtaining better food for their families, driving them to solely purchase organic items. This segment of the population has grown at an exponential rate over the last decade, and their numbers are continuing to rise. According to reports published by BusinessWire, the global organic meat products market is expected to grow from $14.38 billion in 2019 to $20.39 billion in 2023. To meet this market demand, manufacturers must take on the task of producing higher-quality, consistently efficient, value delivering sustainable herbal products.
We explored what standardized botanical powders (SBPs) are, their value, and the benefits they give to the poultry farming community in prior articles in this series. SBPs are herbal powders whose specific phytochemical active concentrations are standardized with minimal variation, to ensure efficient Phytoactive function in the animal’s body. Through standardization of botanical powders, the product can be monitored for consistency, and it provides the expected and desired results for the animals.
Consistency in efficacy is crucial in today’s poultry sector, and the usage of SBPs will help with that. In previous articles, we discussed the scientific foundation to produce SBPs as well as the protocols that must be followed to ensure that the active phytoconstituents remain of high quality. It’s crucial to keep the herbal constituents stable in a poly-herbal composition. Environmental factors such as humidity, air, light, and temperature can affect stability. Stability is further affected by factors such as particle size, pH, the characteristics of water and other solvents used during manufacturing, the nature of the container, and the presence of other chemicals resulting from contamination. Maintaining an SBP’s stability ensures that the product’s strength, quality, and purity remain consistent as per specifications. In the current article, we shall shed light on the desired botanical, organoleptic, physical, chemical, and biological properties of SBPs that make them stable and maintain high quality.

Figure 1: The many assessments that an SBP must go through are depicted in this schematic representation
SBPs should be assessed and documented for their properties
SBPs should be thoroughly studied for their inherent properties as shown in Figure 1.
Botanical properties such as physical shape, external appearance, and markings should be documented.
Organoleptic properties are properties that create an individual experience through the senses of the consumer. These properties are color, odor, texture, taste, and fracture.
Physical attributes such as pH, moisture content, ash value, which are the inorganic residues obtained after complete combustion of a compound, and extractive values are used to assess quality, purity, and to detect adulteration.
Chemical properties should be assessed through high-performance liquid chromatography (HPLC), high-performance thin-layer chromatography (HPTLC), thin-layer chromatography (TLC), and gas chromatography (GC). The SBPs should be analyzed both qualitatively and quantitatively for their chemical properties.
Biological properties should be studied for microbial contamination, toxicological and pharmacological residues.
The next sections go through some of the key attributes that contribute to the making of an efficient SBP.
Assessment of the particle size of the SBPs
Poultry are simple stomached animals largely dependent on the repertoire of endogenous enzymes for their nourishment. One of the most critical aspects that determine feed utilisation in these animals is particle size distribution. Finer particle size provides for better contact with digestive enzymes, which results in optimal nutrient absorption and improved animal performance. However, the fineness of the particle size has limitations. Increased incidences of gizzard dysfunction are seen in the flock when the particle size is very fine. Hence, during the manufacture of SBPs, the particle size of the product plays an integral role. Particle size can be assessed using a particle size analyzer, as in Figure 2, which works on the principle of laser diffraction. Large particles scatter light at small angles as compared to that of the laser beam, whereas small particles scatter light at a much larger angle. The angular scattering intensity is used to determine particle size. The flow of the SBP particles should be assessed for parameters such as the angle of repose, which is a characteristic related to inter-particulate friction or resistance to movement between particles, the compressibility index, and Hausner ratio. In Figure 3, the particle sizes of three products, A, B, and C, are compared. While they are all in powder form, higher magnification reveals that product A has the best particle size when compared to B and C. The ability of a product to be homogeneously blended into the feed mixture is also influenced by particle size. Hence, as indicated in Figure 4, an SBP must be examined for its capacity to be uniformly mixed.

Figure 2: Particle size analyzer results

Figure 3: Particle size of different products shown at higher magnification

Figure 4: Assessment of uniform mixing of the SBP with other feed ingredients
Thermostability assessment
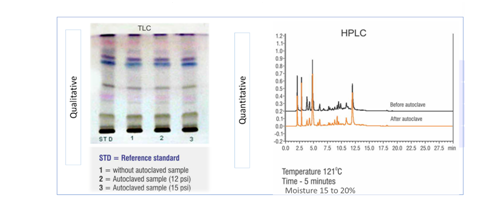
It has been observed that, under higher temperatures, many of the constituents present in poly-herbal formulations may react with each other, raising serious concern about the stability and efficiency of the formulation. Natural products are often susceptible to deterioration, especially during storage, leading to the production of metabolites with no activity, loss of active phytoconstituents, and, in extreme cases, the production of toxic metabolites. Hence, qualitative, and quantitative evaluation of SBP stability at higher temperatures is essential. In Figure 5, the product under test doesn’t show any change in the active ingredient composition before and after being autoclaved at 121͒ C, indicating that the compound is thermostable. These results ensure that the SBP is stable and will have the desired biological activity in the target animal.
Microbial Load assessment
Herbal plants may be associated with a broad variety of microbial contaminants transmitted through soil or air as illustrated in Figure 6. They could be bacteria, fungi, or viruses. Multiple environmental conditions influence the microbial load, which has a significant impact on the overall quality of herbal goods and preparations. According to study reports, the most found pathogens are enterobacteria such as E. coli and Salmonella. Hence, microbial assessment of medicinal plants on procurement and SBPs after manufacturing is essential. ISO guidance suggests standardized protocols for aerobic mesophilic bacteria, yeasts, and moulds, E. coli, and Salmonella in herbal medicines as shown in Figure 7.


Assessment for toxic contaminants
Contaminants such as heavy metals, pesticides, and mycotoxins, if not maintained below safe levels, can lead to life-threatening toxicity in animals. To ensure the SBPs are safe, they should be tested for these contaminants. The samples can be tested for pesticide residues with Gas Chromatography – Electron Capture Detector (GC-ECD) and Gas Chromatography-Tandem Mass Spectrometry (GC-MS/MS). Heavy metal contamination can be detected using an inductively coupled plasma mass spectrophotometer (ICP-MS). Aflatoxin and mycotoxins can be detected using high-performance liquid chromatography with fluorescence detection (HPLC-FLD).
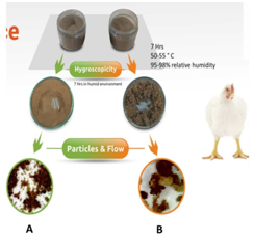
Assessment of Hygroscopicity
The SBPs should be non-hygroscopic, among other physical qualities. The ability of a substance to absorb moisture from its surroundings is known as hygroscopicity. It’s an unfavorable feature since it might cause lumps and prevent correct mixing with other feed ingredients. As a result, the SBPs must be designed to be non-hygroscopic. Figure 8 depicts a method for determining hygroscopicity. Product A is less hygroscopic as compared to B since it doesn’t form lumps.
The efficiency of a feed supplement can be attributed to its physical properties as well as its functional efficacy based on the performance of the birds. Here we elaborately provided evidence of the physical attributes necessary to be tested in an SBP, which would keep the product stable and efficient for a prolonged period as well as make management of the product easy. In our next article, Productivity Check of SBPs in the Field, we shall elaborate on the scientific assessment of functional efficiency through their biological effects.
Natural Remedies is the number 1 veterinary herbal healthcare company in India with presence in more than 30 countries across the globe. Through its world-class Research and Development centre, Natural Remedies offers a category of science-based Phytogenic feed additives, called Standardised Botanical Powders (SBPs). In this series of articles, Dr. Raina Raj, Head of Marketing at Natural Remedies, provides in-depth knowledge of what SBPs are, and their benefits in the poultry diet.









