
INTRODUCTION
Avian mycoplasmosis was primarily described in turkeys in 1905 and in chickens in 1930. There are 23 named species of mycoplasma recovered from avian sources but only two of them are established pathogens for domestic poultry as Mycoplasma gallisepticum (MG), Mycoplasma synoviae (MS) causes ‘Chronic Respiratory Disease’. Mycoplasma pathogens cause upper respiratory and locomotory illness in chickens and other avian species. They are responsible not only for clinical diseases but also for decreased weight gain, lowered feed conversion efficiency, reduced hatchability, and downgrading at slaughter (Bradbury, 2001).
Mycoplasma gallisepticum (MG) infection in the commercial poultry industry is common in many areas. Despite the great efforts by poultry breeding companies made towards eradication of pathogenic mycoplasmas from poultry flocks, Still Mycoplasma gallisepticum infection is of continuing economic concern in commercial broiler breeder chicken flocks. Failure in eliminating the disease in grand parent (GP) stock, it persists in broiler breeders and broilers through vertical transmission. The continued presence of MG in commercial broiler breeder flocks suggests that efforts at eradication were not highly successful. This organism is smaller than common bacteria and larger than viruses, but lacks a cell wall. This characteristic makes MG extremely fragile (no cell wall) and difficult to culture (specialised growth requirement) and host adapted (avian only).
Respiratory tract infections are of great importance in poultry industry, causing heavy economic losses. Mycoplasma gallisepticum and Mycoplasma synoviae are the most pathogenic organisms of the respiratory tract.

Other respiratory tract infections include both viral pathogens (Newcastle disease virus, Infectious bronchitis virus, avian influenza virus) and bacterial pathogens (Salmonella pullorum, Escherichia coli, Avibacterium paragallinarum, etc) cause disease independently and in association with each other and causes Complex Chronic Respiratory Disease (CCRD).
Mycoplasma control for any companies requires integrated approach involving diligent biosecurity, animal husbandry & disease survivallance. The consequences of wide spread infection in breeder operation can be devastating result of both as direct and indirect losses occurring throughout the production cycle (Ley,2003).
TRANSMISSION
MG and MS can spread through horizontally and vertically route of susceptible birds with infected chickens; spread may also occur by contaminated airborne dust, droplets, or feathers (Ley and Yoder, 1997). It can be transmitted through the chicken hatching egg to the offspring. MG has been isolated from the oviduct of infected chickens and semen of infected roosters (Yoder and Hofstad, 1964).
CLINICAL SIGN
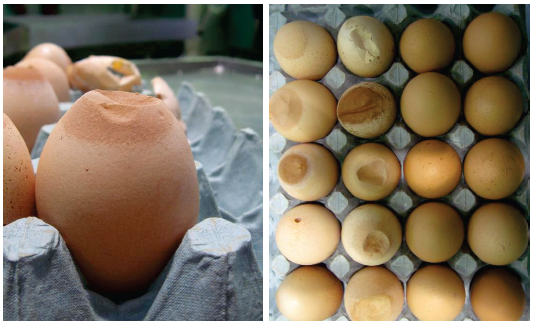
Both diseases are economically important, egg transmitted and hatchery disseminated diseases. They lead to tremendous economic losses in poultry production as a result of decreased hatchability and egg production, reduced quality of day-old chicks, reduced growth rate. Chicken showed swelling of the facial skin, and the eyelids, increased lacrimation, congestion of conjunctival vessels, and respiratory rales.
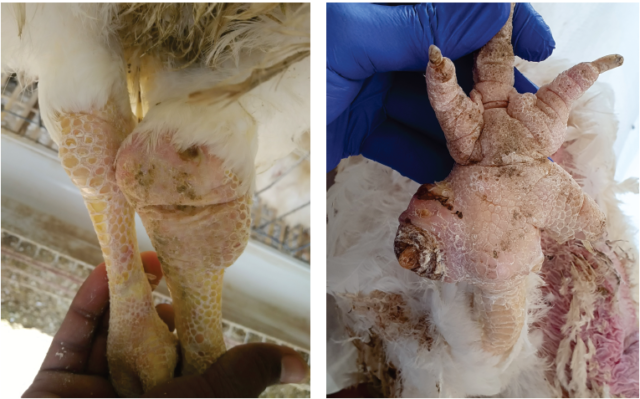
Mycoplasma synoviae (Ms) infection is usually known as infectious synovitis, an acute-to-chronic infectious disease for chickens involving primarily the synovial membranes of joints and tendons sheaths. However, during recent years, MS has less frequently been associated with synovitis but more frequently associated with airsacculitis in chicken.
PATHOGENESIS
It is presumed that MG enters the respiratory tract by inhalation of aerosols or via the conjunctiva and attaches to mucosal cells by its well-organized terminal organelles, which remains and spread in respiratory system.
As MG & MS are exhibiting with no cell wall, it is readily killed by most of the disinfectants, heat, and sunlight, and does not survive for prolonged periods outside the host. MG can remain viable
- Chicken faeces for 1-3 days at 20°C,
- Muslin cloth 3 days at 20°C or 1 day at 37°C,
- 3. in egg yolk 18 week at 37°C or 6 week at 20°C. It only remains viable in the environment, outside the chicken, for typically up to 3 days. For this reason, MG is fairly easy to eliminate on single age, all-in all-out poultry farms Since MG can be transmitted vertically. Establishing the MG-clean status of breeder flocks and maintaining that status can be accomplished by participation in control programmes. An MG eradication programme may be initiated by treatment of breeders and their hatching eggs to reduce egg transmission. Attempts to eliminate egg transmission of MG by medication of breeder flocks or their progeny with antimycoplasmal prevention drug have generally been able to produce considerable reduction in rate of MG infection but generally were not adequate to obtain entirely infection-free flocks. Previously successful methods were the treatment of hatching eggs with heat and/or antimycoplasmal. For heat treatment eggs were gradually heated in a forced-air incubator to reach an internal temperature of 46.10C over 12-14 hour and then allowed to return to room temperature (Yoder, 1970). Hatchability was sometimes reduced 8-12%, but MG and MS appeared to be inactivated. Egg dipping with a temperature or pressure differential has been used by several researchers as a means of getting antibiotics into hatching eggs to eliminate egg transmitted MG (Alls et al., 1963;Hall et al., 1963; Stuart and Bruins, 1963).
LOSSES CAN OCCURS AS RESULT OF
- Decreased egg production
- Decreases egg hatchability
- Decreased day old chick quality and chick viability 4. Increase chick mortality
- Higher FCR and low weight gain
- Costly control measures involving biosecurity, vaccination & medication.
Control of pathogenic avian mycoplasma can consist of one of three general approaches, according to Kleven (2008): The mycoplasma infection are transmitted both horizontally and vertically and it’s remained in the flock constantly as sub clinical form. To control MG infection in broiler breeder, laying hens and commercial broilers chicken the major specific focus is given on vaccination and medication. - MAINTENANCE OF FLOCKS, WHICH ARE FREE OF INFECTION.
To keep a flock free of infection is difficult, especially in areas where large populations of chickens have grown up, as the industry has expanded. To maintain freedom from mycoplasma requires a mycoplasma free source, on a single age, ‘all in all out’ site, with good biosecurity and an effective monitoring system. - CONTROL BY VACCINES.
The use of mycoplasma vaccines in breeding & laying hens has grown over recent years to reduce the impact of infections, but these can confuse the usual serological monitoring systems. They may control an infection in the chicken clinically but there is still a potential risk of vertical transmission to the egg and chick. Vaccination could not completely prevent the occurrence of EAA, although a significant reduction of EAA egg production (approximately 50%) was recorded. Moreover, a delay in the onset of egg production was observed in the vaccinated birds (Feberwee et.al. 2009).
- KILLED/INACTIVATED VACCINES
➤ These are M. gallisepticum killed organisms with oil emulsion adjuvants to protect the birds from infection with virulent M. gallisepticum.
Several adjuvant enhanced bacterin vaccines but they are expensive and administration is difficult because they need to be injected twice with a 4-6 week interval (Ley, 2003).
Killed vaccines have been shown to reduce, but not eliminate the M. gallisepticum infection and are not effective in long term control of infection in multiple age farms.
➤ Killed vaccination did not reduce horizontal spread of M. gallisepticum (Levisohn et. al., 2000).
➤ These are more stable and safer than live vaccine.
- LIVE/ATTENUATED VACCINE
➤ There is three type of live vaccines is available for M. gallisepticum viz.
A. CONNECTICUT F-STRAIN
B. MG 6/85 STRAIN
C. TS-11 STRAIN (TEMPERATURE SENSITIVE MUTANTS)
A. CONNECTICUT F-STRAIN
➤ Live F-strain M. gallisepticum vaccine is a relatively mild strain that originate from the Connecticut F strain of United States. Despite the advantages of the f-strain vaccine it has many of the disadvantages of the inactivated vaccines.
MG free chickens tend to lay better than F-strain immunised ones.
➤ F-strain is too virulent for young chicks.
➤ F-strain is capable of lateral spread in the flock.
F-strain does not completely block trans ovarian transmission when birds are challenged with virulent MG.
B. MG 6/85 STRAIN
The 6/85 strain of MG is in lyophilised form and originate from United States.
It has low virulence in chicken.
Vaccinates were protected against airsacculitis and colonisation of the trachea was detectable from 4 to 8 weeks after vaccination (Ley, et. al., 1997).
C. TS-11 STRAIN
ts-11 is a live chemically induced mutant strain of MG is in frozen form and developed from Australian MG field isolate (Whithear et. al., 1990a).
- CONTROL BY SPECIAL ANTIBIOTICS
Medication of a flock but can prevent subsequent losses in breeders & laying hens. MS Infections could be treated with antimicrobial use in breeders, layers flock and eggs to prevent vertical transmission.
Control of MG and MS infection in broiler chicken by medication is the most practical way to minimize the transmission of disease and economic losses.
The most important macrolide agent used for treatment and control of mycoplasma infection is Tilmicosin Phosphate.
➤ Tilmicosin is a broad-spectrum bacteriostatic synthesized from tylosin molecule which is having 75 percent more intra alveolar concentration in the lungs tissue to work efficiently against mycoplasma as organism remain intracellular in the cell and tissue.
IN BROILER BREEDER & COMMERCIAL LAYERS
➤ It is very important to treat chicks from day first of life to combat against mycoplasma, Tilmicosin Phospate-25% @ dose rate of 15-20mg/kg body weight through drinking water for 3 successive days every 5 weeks up to for 16th to 20th weeks.
After 20th or 24th week incorporate Tiamulin through feed as per recommendation of veterinarian.
It is emphasized to follow best antimycoplasmal drug prevention programme through feed.
IN COMMERCIAL BROILERS
It is suggested to use Tylosin Tartarate 100% through drinking water for first 3 days @ dose rate of 65 mg/kg of BW.
In high risk or known source of infected breeders it is suggested to use Tilmicosin Phosphate-25% through drinking water for first 3 days @ dose rate of 15mg/kg of BW.
The medication can be repeated on a monthly, three weekly or two weekly basis depending on the mycoplasma status of the flock or the ‘risk’ of breakdown from the proximity of infected neighbours.











