
Embryonic development of the reproductive system
In contrast to the situation in mammals, the sex chromosomes in male birds are ZZ (homozygous) compared to ZW (heterozygous) in females. In males, the two testes are internal and accessory organs such as the prostate and seminal vesicles are absent. The testes develop due to gene dosing with increased expression of the Z-linked transcription factor gene, doublesex and mab-3-related transcription factor 1 (DMRT1). Anti-Müllerian hormone (AMH) is synthesized and secreted by the embryonic testis with greater expression in the embryonic testes than the ovaries. AMH directs the regression of the paired Müllerian ducts.
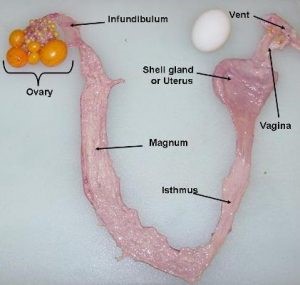
In females, only the left ovary and oviduct develop in all avian species and closely related dinosaurs; the latter based on fossil evidence from the early Cretaceous period. The avian oviduct is derived from the embryonic Müllerian duct; the former term encompassing the entire reproductive tract from infundibulum to the cloaca. Regression of the right oviduct is induced by AMH. Parenthetically, AMH also plays an important role in development of tubules in the testes. The embryonic female gonad expresses the rate-limiting enzyme for the production of estrogens, aromatase (CYP19A1) but expression is not found in the embryonic male gonads. In turn, the estrogens, such as estradiol, induce growth of the oviduct.
Egg development
The egg is comprised of the yolk, yolk membranes, egg white, shell membranes and finally the egg shell. Each of these components are developed along specific regions of the female reproductive tract together with the ovary.
Yolk
The egg yolk is a mature ovum (oocyte) that is produced by the ovary. The maturation of the ovum involves multiple processes including the deposition of proteins/lipids. Yolk protein/lipoproteins/phosphoproteins were assigned to three categories based on centrifugation of diluted yolk:
- Low-density fraction with a very high lipid composition
- Granules are composed of heavy and light chain lipovitellins, phosvitin and a yolk glycoprotein.
Soluble proteins.
The soluble proteins consist of the following:
- α livetins (serum albumen)
- β livetins (serum α2-globulin containing transport proteins)
- γ livetins (serum γ -globulin predominantly immunoglobulin Y).
Egg yolk livetins (α, β, and γ-livetin) have recently been shown to exert anti-inflammatory properties.
Yolk precursors: Yolk precursors are synthesized in the liver. Two major yolk precursors are very-low-density lipoprotein (VLDL) and vitellogenin. Very-low-density lipoprotein (VLDL) has the following characteristics:
- Globular micelle-like
- Non-polar core of triglycerides and cholesterol esters
- Coated with an amphiphilic mix of a phospholipid, free cholesterol (FC) and two apolipoproteins. Chicken vitellogenin has been purified from plasma of estrogen-treated adult male chickens. It is a dimer with a molecular weight 480,000. It is a dimer composed of two polypeptide monomers each with a molecular weight of about 170,000. There are about 220–235 phosphate moieties per monomer vitellogenin and the lipid component is about 20%. Hepatic expression of vitellogenin is induced by estrogens.
Yolk deposition: A specific receptor is responsible for transfer of vitellogenin and very-low-density lipoprotein (VLDL) across the oocyte plasma membrane to fill the oocyte with yolk. Within the oocyte, vitellogenin is cleaved proteolytically to form the yolk proteins, heavy and light chain lipovitellin (20% lipid), phosvitin and a yolk glycoprotein. These are incorporated into yolk granules. Deposition of γ livetins is very high in small follicles <200 mg, but decreases during development of large follicles. For the last four days of development of the follicles, yolk is being deposited at 2.5 cm3 or greater per day.
Once the ovum (egg yolk) has matured, ovulation is stimulated by the pituitary hormone, luteinizing hormone (LH). An extensive explanation of hormonal control of female reproduction follows. If ovulation is successful the ovum is normally received into the infundibulum.
Egg white
The egg white or albumin of the egg is produced by the magnum of the oviduct. The magnum is the longest section of the oviduct where the ovum spends approximately 4 h accumulating egg white proteins.
Among the constituents of egg white are the following proteins:
- Ovalbumen – 50% of egg white proteins
- Ovotransferrin (conalbumen) 12% (this chelates metal ions particularly iron)
- Ovomucoid -11%
- Lysozyme -3.5%
- Ovomucin 1–3%
- Avidin 0.05%.
Antimicrobial peptides and proteins are present in the egg white and include the following:
- Gallin or ovodefensin
- β-defensin 11
- Cathelicidin
- Cystatin – a cysteine protease inhibitor
- Lysozyme-a bacteriolytic enzyme
- Ovoinhibitor.
Eggshell membranes
Following albumin deposition and addition of water (“plumping fluid”) to the developing egg, the eggshell membrane is added in the isthmus; this taking approximately 1 h. The eggshell membranes are 93% protein contains proteins including collagens, ovoalbumin, bacteriolytic enzymes such as ovotransferrin and lysozyme together with clusterin peptides and ovodefensins/defensins such as gallin. These are also glycosaminoglycans including galactosaminoglycan.
Egg shell
The formation of the egg shell in the uterus/shell gland is the final yet it is of longest in duration taking approximately 19 h. This is due to the extensive structure of the shell. The egg shell is 97% inorganic (calcium carbonate). Of the remaining 3% (the decalcified egg shell) is 79% protein with the matrix phosphoproteins including the following: ovocleidin-17, ovocleidin-116, ovocalyxin-32 and osteopontin. The fully formed egg is retained in the shell gland just distal to the vagina of the oviduct until oviposition.
Hormonal control of reproduction
Hormones are critically important to the optimal functioning of the gonads, the photoperiodic stimulation of reproduction, sexual and maternal behavior and induced molting.
Pituitary gland and reproduction: The gonads are controlled by the anterior pituitary hormones, LH and follicle stimulating hormone (FSH). These gonadotropins play a critical role in the development and maintenance of the gonads. FSH increases proliferation of granulosa cells, expression of both steroidogenic acute regulator (StAR) and inhibin α genes in granulosa cells, and release of progesterone with the effect progressively greater with tissue from larger follicles. In addition, prolactin can exert an inhibitory effect on the chicken ovary.
Hypothalamic control of gonadotropin release: There are two gonadotropin releasing hormones (GnRHs) in the chicken (cGnRH-I and cGnRH-II) and two receptors for GnRH (cGnRHR1 and cGnRHR3). GnRH-II is much more potent than GnRH-I in hens in stimulating LH release by 36 fold. However, GnRH-II is not detected in the median eminence. There is high expression of GnRHR3 in the pituitary gland. Therefore, the releasing hormone for LH is chicken GnRH-I and the receptor is cGnRHR3.
Chicken gonadotropin-inhibitory hormone (GnIH) is a peptide with 12-amino-acids. While GnIH inhibits both the synthesis and the release of gonadotropins in chickens, the physiological relevance of GnIH still requires clarification.
The ovary produces the following:
- Estrogens, primarily estradiol. Estrogens induce the following: development of the oviduct, production of yolk precursors (VLDL and vitellogenin) (see above) by the liver, production of egg white proteins by the oviduct and, with androgens, formation of medullary bone (a labile source of calcium). In addition, estrogens allow the expression of female behaviors and moderate the release of luteinizing hormone (LH).
- Progesterone. Among its roles are stimulating the production of a specific egg white protein (avidin) and stimulating the release of LH.
- Androgens, predominantly testosterone. Androgens are essential to the development of medullary bone.
Ovarian hormones and growth factors also play critical roles in follicular development. For instance, activin A increases the expression of both FSH and LH receptors but decreases cell proliferation of granulosa cells. Moreover, the development of small follicles is suppressed by epidermal growth factor receptor ligands such as transforming growth factor α. In contrast, bone morphogenetic protein 6 enhanced responsiveness to FSH.
Light and reproduction
Photoperiodic induction of reproduction
There is seasonality of egg production in chickens when held under a natural photoperiod in the temperate zone. Egg production increases markedly after the winter solstice and declines beginning prior to the autumnal equinox. The physiological basis of this annual cycle is photoperiodic stimulation of reproduction by long daylengths; these inducing the development of functioning gonads. Red light is detected by photo-pigments in the hypothalamus, with the most important photoreceptor influencing the hypothalamic release of GnRH-I being red opsin. The photoperiodic mechanism involves light coinciding temporarily with the light-sensitive (photo-sensitive) phase of a circadian rhythm. This leads to the release of GnRH-I, synthesis and secretion of LH and FSH and, hence, gonadal resurgence.
Chickens Pullets are reared under short daylengths (6L:18D or 8L:16D). They are transferred to longer daylengths (12L:12D) at breed-specific physiological ages so stimulate gonadal development. In studies where pullets were transferred to daylengths of 10L:14D or 11L:13D, plasma concentrations of LH did not increase, but marked increases in plasma concentrations of LH daylengths were observed with daylengths 13L:11D or greater. Perhaps surprisingly, daylengths were interpreted differently depending on the previous photoperiod. Transfer of pullets from photoperiods of either 4L:20D or 20L:4D to 12L:12D were followed by, respectively increases and decreases in plasma concentrations of LH. Thus, the same photoperiod can be interpreted as either photostimulatory or photoinhibitory.
Photorefractoriness and reproduction
Photorefractoriness is the loss of the ability to respond to the stimulatory effects of long photoperiods. Photorefractoriness can be “broken” by re-exposure to short day-lengths. This is seen in turkeys with prolonged exposure to long day-lengths with the signs of photorefractoriness being decreased egg production and molting. The decline in egg production during the production cycle of chickens might also be attributed to photorefractoriness. Indeed, there is greater sensitivity of older hens to reduced daylength with an over 90% decrease in egg production in 105 weeks old hens compared to a 26% decline in 28 weeks old hens. Moreover, plasma concentrations of LH were only decreased in the older hens.
Light intensity and poultry reproduction
While, light intensities greater than 1 lux are required for photoperiodic induction of egg production, considerably higher light intensities are employed in commercial poultry production. For instance, in broiler breeders, light intensity is increased from before photostimulation about 6 lux in the pullet phase to >50 lux after photostimulation at 21 or 22 weeks of age.
Other effects of light intensity: Light intensity has other effects. For instance, increasing light intensity in immature pullets is associated with increased plasma concentrations of FSH. Moreover, the ability of a short pulse of light to photo stimulate chickens is influenced by light intensity. In addition, the ratio of the light intensity during the subjective day to that during the subjective night is important in entraining the rhythm of oviposition.
Nutrition and reproductive management
In poultry, nutrition is integrally linked to the hypothalamo-pituitary-gonadal axis. It has been known for 50 years that egg production in hens stops quickly following fasting. The administration of mammalian or avian gonadotropin restores, albeit partially, egg production in starved hens; this suggesting that underlying cause is the lack of pre-ovulatory LH surges. Fasting is followed rapidly by decreases in plasma concentrations of LH, body weight together with precipitous declines in ovarian and oviductal weights. Similarly, production of eggs and plasma concentrations of LH decrease quickly after reducing calcium or sodium in the diet of hens. In young chickens, protein deficiency also has been demonstrated to rapidly cause atrophy of gonads, decrease circulating concentrations of LH and depress responsiveness to GnRH.
Induced molting or re-cycling to increase egg production
Hens can be induced (or forced) to molting at the end of their laying cycle resulting in improved egg production at a lower cost than using replacement pullets. In the USA, 19.7 % of laying hens are molted (re-cycled) each month. This process can involve severe nutritional restriction including starvation and/or withholding water and/or reduction in photoperiod. Alternate methods of induced molting include an extremely high zinc diet (20,000 ppm) followed by a conventional layer feed beginning at day 12 and sodium/chloride-deficient diets. The terms, forced or induced molt, are open to question as it presumes that molting (loss of feathers) causes rejuvenation of reproduction performance.
Molting occurs after resumption of normal feeding and is temporally shifted from ovarian recrudescence. When feed is withdrawn for 8 days and water withdraws for 2 days, egg production had completely ceased by 6 days. Molting occurred after the resumption of feeding and there were concomitant increases in circulating concentrations of T3 and corticosterone Circulating concentrations of LH, estradiol (E2) and progesterone were lower in molting hens than in laying hens or fully recycled hens.
The physiological mechanism underlying induced molting included decreased release of GnRH from the median eminence and consequently lack of the pre-ovulatory LH surge. Ovulation completely ceased with 4 days of feed withdrawal. Plasma concentrations of LH and progesterone were decreased with 2 days of feed withdrawal. The GnRH content of the median eminence was similarly decreased but not until 4 days of feed withdrawal. There are also decreases in the number of gonadotropes expressing LH. Oviductal regression occurs due to lack of estrogens and is accompanied by increased expression of peptidases with, for example, expression of the peptidase, cathepsin L.
Authors:

1* AKANKSHA GUPTA, 2 Shubham Thakur 3 Krishna Nand Bansal 4 Usha Yadav
1* P.hd scholar Department of Dairy Cattle Physiology, National Dairy Research Institute Karnal, 132001.
2 P.hd Scholar Department of Animal Nutrition, , Lala Lajpat Rai University of Veterinary and Animal Sciences, Hisar, 125004.
3 M.V. Sc Scholar, Department of Veterinary Gynecology and Obstetrics, Lala Lajpat Rai University of Veterinary and Animal Sciences, Hisar, 125004.
4 M.V. Sc Scholar, Department of Veterinary Gynecology and Obstetrics, Rajasthan University of Veterinary and Animal Sciences, Bikaner, Rajasthan, 334001









