Mycoplasma spp., identified up to now, (M. Gallisepticum, M. Synoviae, M.iowae) have been negatively affecting commercial poultry production for many years. The poultry industry and scientific community have made great strides in increasing the knowledge of the biology of these bacteria since they were first identified, but much is still to be revealed.
Mycoplasmas are small bacteria that lack a cell wall and certain metabolic pathways, both important targets for antibiotics. This is important to remember when choosing an antibiotic for control or treatment. Mycoplasmas were often considered to have a limited survival time outside the host.
However, some recent data show that animal mycoplasma species can survive for variable time periods outside the host, depending on the species, moisture, pH, presence of organic material, and temperature. Some species have been shown to survive for 50-150 days at 4˚C in liquid media and from 7-14 days under dry conditions at 30˚C. Recently M.synoviae was shown to survive for nine days on synthetic materials.
The presence of persistently infected populations (backyard and wild birds) ensures that the biosecurity of surrounding flocks is continually challenged. These are important reasons why mycoplasma is still a major problem in the poultry industry. Secondly, antigenic variation and intracellular location of Mycoplasma spp. help the pathogen to evade the immunity system, leading to chronic infected animals and the fact that vaccines can only help, in the best case scenario, to reduce production losses and clinical symptoms.
The current approaches to control avian mycoplasma include continuous surveillance and quarantine measures, medication, vaccination and/or elimination of infected breeding flocks. To maintain mycoplasma-free flocks it is important to use only negative replacements, use single age farms (isolated if possible), depopulate and disinfect between flocks, maintain good biosecurity, and set up a monitoring program.
Elimination of a positive breeder flock is the surest way to eliminate the shed of M. gallisepticum or synoviae, but this is not always feasible. Positive flocks should be isolated as much as possible; the eggs and chicks should also be segregated. Once a flock is infected or vaccines are unable to control mycoplasma, antibiotics are still required.

Table 1. Antibiotic susceptibility surveys showing limited resistance to M.gallisepticum and synoviae for tylosin (Pharmasin), tilmicosin (Tilmovet), and tiamulin (Vetmulin).
The clinical outcome of this antibiotic treatment depends on three crucial steps in the decision process of the veterinary surgeon: Selecting the correct antimicrobial, considering: Known or suspected antimicrobial susceptibility of the pathogen. Ability of the antimicrobial to sufficiently reach the site of infection.
- Other features.
- Correct dosing and administration.
- Product choice, with a bioavailable/potent active compound and an appropriate formulation.
Selecting the correct antimicrobial
The susceptibility of a pathogen can be based upon susceptibility testing, which is, unfortunately, rather complicated and time demanding for Mycoplasma spp. For this reason, the clinical experience of the veterinarian, farm history and antibiotic susceptibility surveys (Table. 1) are also of importance.

Fig. 1. Pharmacokinetic behaviour of Tilmovet 250mg/ml after three days of treatment (day 1, day 2 and day 3) at 15mg/kg bodyweight. Levels in lung and airsacs stay above MIC90for at least eight days.
In addition to the susceptibility outcome, the antibiotic needs to reach sufficient concentrations in the respiratory tract and preferably also be present intracellularly (as mycoplasmas are located intracellularly). Pharmasin (tylosin), Tilmovet (tilmicosin) and Vetmulin (tiamulin) not only deliver high concentrations in the respiratory tract (Fig. 1), but also show beneficial intracellular/extracellular ratios of up to 75. Other features are also of importance when choosing the right antimicrobial to treat and control mycoplasma. Some products are better suitable for layers (Pharmasin), whilst others are more suited for breeders (Vetmulin) or for start-up (Tilmovet). Pharmasin , for example, does not have any negative effect on water intake, is very safe and with no known incompatibilities. Moreover, Pharmasin has a zero withdrawal time for eggs in the EU, which makes the product ideal for the control and treatment of Mycoplasma spp. in layers. Vetmulin has a very unique feature: it ensures that concentrations in the eggs remain above the MIC90 for both M. gallisepticum and M. synoviae for several days, which is the reason why excellent results are achieved to control vertical transmission in breeder stocks in the field. The slow elimination phase of Tilmovet (Fig. 1) results in prolonged continuous tissue concentrations, making it less dependent on variable feed and water intake. Some antibiotics are known to have a negative influence on the immunity build-up, possibly interfering with vaccination response. On the contrary, the macrolides and specifically Tilmovet, have been shown to have a positive effect, making the product ideal for start-up and for pullets.
Correct dosing and administration
After choosing the ideal antibiotic based upon susceptibility, pharmacokinetic behaviour and additional features, a correct administration is features, a correct administration is also of critical importance. Dosing should be done in grams per kilogram live body weight, independently of the application form. By doing so, misdosing will be avoided by taking into account the changing ratio of body weight/water; or feed intake, which is especially important in fast growing birds, such as broilers. Correct dosing in mg/kg body weight can easily be achieved with the Huvepharma Dose Calculator, freely available for iPhone and Android mobile devices.
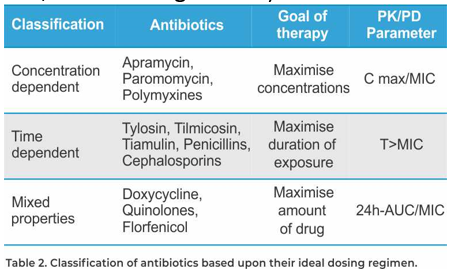
In addition to the dosage per kg body weight, the dosage regimen is also of importance. A daily dose can be administered in different ways, either continuously or as a pulse. For time-dependent antimicrobials, such as Pharmasin (tylosin), Tilmovet (tilmicosin) and Vetmulin (tiamulin),the efficacy is determined by the period during which the bacteria are exposed to the antimicrobial at a concentration just above the MIC (T>MIC).
The most important parameter is the time period in which the concentration is higher than the MIC (T>MIC) at the site of infection. For this reason, the highest efficacy can be expected if these antimicrobials are administered continuously over 24 hours, for a sufficiently long period.
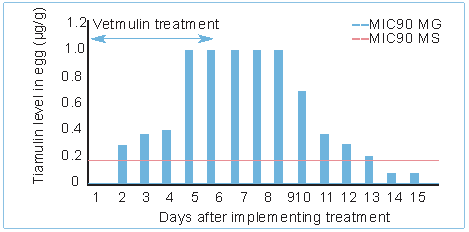
Fig. 2. Concentration of tiamulin (Vetmulin) in the egg, during and after treatment.
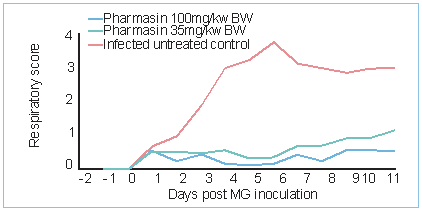
Fig. 3. Mean respiratory score of M. gallisepticum challenge study with different dose levels of Pharmasin.
The most important parameter is the time period in which the concentration is higher than the MIC (T>MIC) at the site of infection. For this reason, the highest efficacy can be expected if these antimicrobials are administered continuously over 24 hours, for a sufficiently long period. For concentration-dependent antibiotics, for example apramycin, a high concentration (Cmax) several times higher than the MIC of the targeted pathogen at the site of infection, will result in a faster and better response. For these antimicrobials, the most important parameter is the Cmax/MIC.
Consequently, a pulse medication will work better for these types of antimicrobials. Mycoplasma efficacy studies with Pharmasin, Vetmulin and Tilmovet indicate that therapeutic levels for a minimum of five days are appropriate. For this reason, a minimum treatment period of five days is recommended for Pharmasin and Vetmulin and of three days for Tilmovet. Depending on the risk of exposure, the treatment can be repeated every four weeks (low risk) up to every two weeks (high risk, like multi-age farms).
Product choice
The formulation of the veterinary product will also influence the clinical outcome of an antimicrobial treatment. Stability, solubility and bioavailability of the active compound can be optimised by the choice of a correct product (brand). The absorption and distribution rate of a product in the body has a direct and critical impact on the clinical outcome of the treatment.
Often, veterinary products containing the same amount of active substance are considered as equivalent. However, the behaviour of a pharmaceutical product depends on several product features such as:
Quality of the active (crystal form and size, impurities, presence of undesired substances such as heavy metals). Choice and quality of the salt (for example: tartrate, phosphate or hyclate).
Formulation: used excipients and type of formulation (simple mixture, carrier or granulated).
In vivo studies, although time consuming and expensive, can confirm the efficacy of the products at different dosing regimens after challenge with the pathogen.
The most important parameter is the time period in which the concentration is higher than the MIC (T>MIC) at the site of infection. For this reason, the highest efficacy can be expected if these antimicrobials are administered continuously over 24 hours, for a sufficiently long period. For concentration-dependent antibiotics, for example apramycin, a high concentration (Cmax) several times higher than the MIC of the targeted pathogen at the site of infection, will result in a faster and better response. For these antimicrobials, the most important parameter is the Cmax/MIC.
Consequently, a pulse medication will work better for these types of antimicrobials. Mycoplasma efficacy studies with Pharmasin, Vetmulin and Tilmovet indicate that therapeutic levels for a minimum of five days are appropriate. For this reason, a minimum treatment period of five days is recommended for Pharmasin and Vetmulin and of three days for Tilmovet. Depending on the risk of exposure, the treatment can be repeated every four weeks (low risk) up to every two weeks (high risk, like multi-age farms).
Product choice
The formulation of the veterinary product will also influence the clinical outcome of an antimicrobial treatment. Stability, solubility and bioavailability of the active compound can be optimised by the choice of a correct product (brand). The absorption and distribution rate of a product in the body has a direct and critical impact on the clinical outcome of the treatment.
Often, veterinary products containing the same amount of active substance are considered as equivalent. However, the behaviour of a pharmaceutical product depends on several product features such as:
Quality of the active (crystal form and size, impurities, presence of undesired substances such as heavy metals).
Choice and quality of the salt (for example: tartrate, phosphate or hyclate).
Formulation: used excipients and type of formulation (simple mixture, carrier or granulated).
In vivo studies, although time consuming and expensive, can confirm the efficacy of the products at different dosing regimens after challenge with the pathogen.
Results from these trials allow for a more cost-efficient, more efficacious and more sustainable use of products, which is especially important when justifying antimicrobial therapy. The efficacy of Pharmasin to control mycoplasma was tested at different dosing levels (Fig. 4). Broilers (n=45) were kept in isolators and challenged with a M. gallisepticum isolate (Italy, 2012, MIC value <0.015µg/ml).
The treated groups were given 35 and 100mg tylosin/kg body weight respectively for five days, starting one day post-challenge. The control group was infected but did not receive treatment. Monitored parameters were, amongst others, clinical scoring of respiratory disease, macroscopic scoring of the respiratory tract, weight gain, mortality and M. gallisepticum recovery from trachea, airsacs and lungs.
Both dosing levels were efficacious in protecting against the detrimental consequences of M. gallisepticum infection as indicated by the difference with the infected untreated control group. Despite fine-tuning of management, vaccination schemes, feeding, housing and biosecurity, animals can still become diseased. This is why antibiotics are, and will stay, essential for protecting animal health and welfare as well as the safe production of food of animal origin. However, responsible and wise use of medicines is mandatory to safeguard the use of veterinary medicines in the long term. This means targeting the pathogen with the right product and administering it correctly.
One such major pathogen is mycoplasma, for which Huvepharma can offer the right tools and the right advice based upon extensive field experience and product-specific efficacy trials.
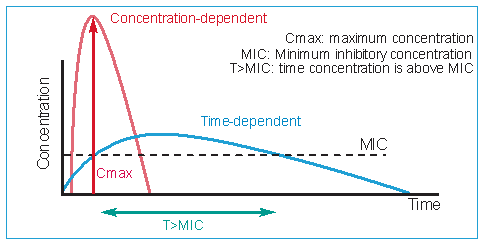
Fig. 4. Two types of antibiotics with an ideal pharmacokinetic profile in regards to efficacy.
To know more, please contact Huvepharma technical team

Huvepharma SEA (Pune) Pvt. Ltd.
42, ‘ Haridwar’, Road 2 A/B, Kalyani Nagar, Pune 411006
Customer Care Contact: +91 20 2665 4193 Email: salesindia@huvepharma.com
Website: www.huvepharma.com
Author:
WOUTER DEPONDT, HUVEPHARMA, BULGARIA