
Repeat Breeding Syndrome (RBS): When a normal cyclic female animal with apparently normal genitalia, normal estrous cycle (inter-estrus length 21-22 days), normal estrus period (18-24h) and normal cervico-vaginal mucus discharge, fails to conceive when mated in three or more consecutive estruses with fertile bull or inseminated artificially with fertile semen is called as Repeater/Repeat breeder/ Cyclic non-breeder animal and the condition is termed as Repeat Breeding.
- The condition is multifactorial in origin so refereed as Repeat Breeding Syndrome (RBS).
- Incidence of RBS in a herd is around 5-30% (average 10-15%) and it could be more in solitary animal rearing.
- The causes of RBS are mainly categorized under two headings:
- Failure of fertilization
- Early Embryonic Mortality (EEM)
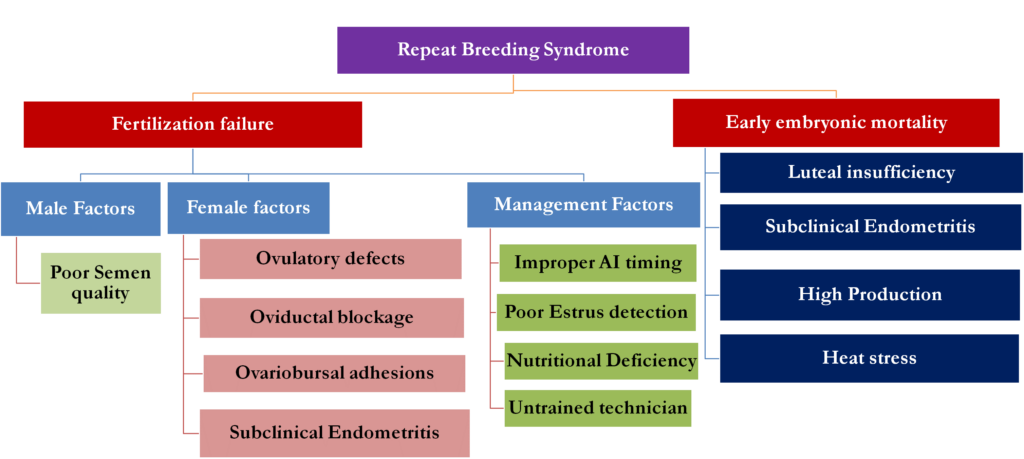
Fig. 1: Various causes of repeat breeding syndrome in dairy animals
1. Failure of fertilization: The fertilization rate in normal cases is more than 90% in dairy animals but, there are some animals which have some abnormalities due to which fertilization don’t take place and animal comes in estrus again after a period of 21-22 days.
2. Early Embryonic Mortality (EEM): When after proper fertilization, the embryonic survival is impaired (embryo dies) before the time of maternal recognition of pregnancy (MRP) resulting in luteolysis at 16-17 days post estrus and the animal comes in heat at a regular interval without affecting the length of estrous cycle, it is termed as EEM. The incidence of EEM could be up to 25-30% in a herd.
- Failure of Fertilization
The failure of fertilization could occur due to
- Male factors
- Female factors
- Management factors
A. Male factors: The failure of fertilization could be due to use of infertile bull for mating or semen collection, high sperm abnormalities, poor post-thaw motility of semen due to lowlevel of liquid nitrogen in the semen storage containers. Therefore, all these factors leading to poor sperm health and can lead to fertilization failure, so, checking of the semen quality and use of only fertile good quality semen is necessary to ascertain fertilization.
B. Female Factors: There could be many factors related to female animal which could lead to failure of fertilization including ageing of ovum, failure of meeting of sperm and oocyte, failure of implantation etc. The major female associated factors are:
- Ovulatory defects: The failure of ovulation which is also referred as anovulation, delayed timing of ovulation are major ovulatory defects due to which spermatozoa ageing occurs as well as there is reduced quality of oocyte in the delayed ovulation cases. One cause of delayed or anovulation is suprabasal progesterone at the time of ovulation due to improper luteolysis of the corpus luteum. Another cause of ovulatory defect could be lesser concentration of GnRH and LH hormones which are required for late follicular maturation and ovulation.
- Oviduct blockage: The oviduct is the route for the transport of the gametes as well as site of fertilization, so, any blockage of oviduct shall impair the fertilization process either due to impaired transport of gametes or zygote. The oviduct blockage could occur due to salpingitis (inflammation of oviduct), hydrosalpinx (accumulation of watery fluid in oviduct), pyosalpinx (pus in oviduct) or pachysalpinx (connective tissue in oviduct).
- Ovario-bursal adhesions: The bilateral ovario-bursal adhesions make the animal sterile. Unilateral ovario-bursal adhesions ipsilateral to ovary undergoing ovulation lead to repeat breeding.
- Subclinical/Cytological endometritis (SCM): The inflammation of endometrium due to infiltration of polymorphonuclear cells (PMNs)/ neutrophils in the lumen of the uterus is termed as SCM. The incidence of SCM could be up to 25-40% and lead to failure of implantation and RBS.
C. Management Factors: Various human associated factors responsible for fertilization failure & ultimately RBS are:
- Improper heat detection
- Environmental stress
- Nutritional deficiencies
- Improper timing of AI
- Untrained inseminator/faulty AI
- Unhygienic conditions at the time of AI
- Improper thawing of straw
2. Early Embryonic Mortality (EEM)
The major causes of EEM are:
- Luteal insufficiency: The adequate progesterone concentration is mandatory for the embryonic survivability as under the luteal environment proper implantation establishes as well as early nourishment of embryo occurs by uterotroph (uterine milk) which is secreted by endometrium under influence of progesterone. The luteal insufficiency can occur either due to poor development of corpus luteum (CL), lesser production by CL or early luteolysis of CL. The buffaloes are more prone to luteal insufficiency due to smaller CL size and lesser number of luteal cells in CL. Thus, the lower concentration of progesterone will result in EEM and RBS.
- Subclinical endometritis: Presence of subclinical endometritis leads to failure of implantation of embryo and thus, RBS.
- Nutritional deficiencies: Deficiency of wide range of specific nutrients has been observed in poor reproductive performance. Particularly, Vitamin E and Selenium are reported to cause early embryonic death. Feeding of estrogenic forages to the cows and buffaloes also affects the embryonic survival.
- Stress: Elevated temperature due to persistent fever or high environmental heat and humidity may lead to the early embryonic death. As well, the high yielding animals have lactation stress which leads to poor oocyte quality and poor embryonic development and ultimately EEM and RBS.
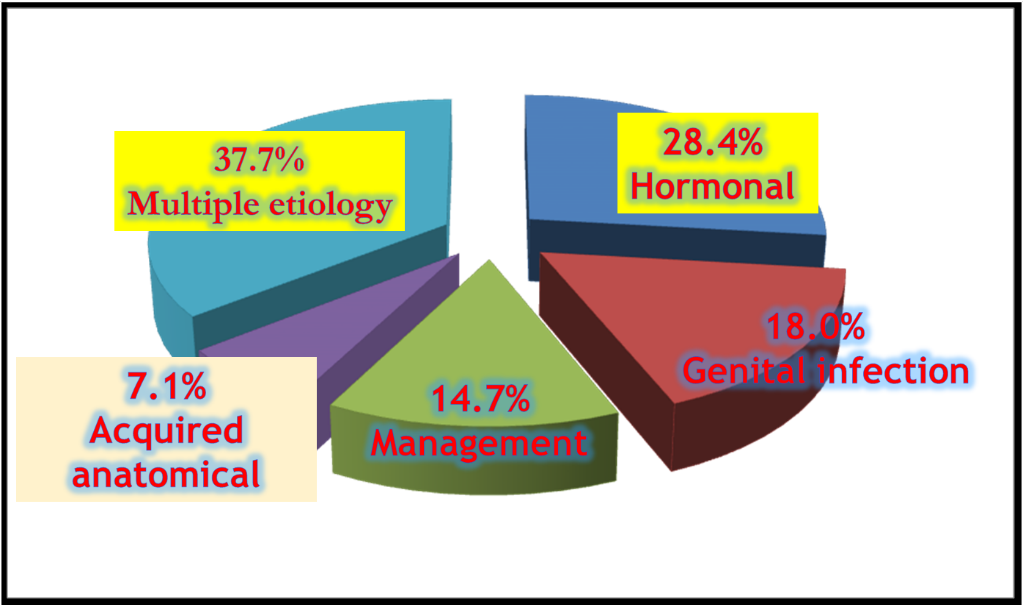
Fig. 2: Incidence wise female causes of RBS in cows
Diagnosis of RBS
The diagnosis of proper cause of RBS depends upon the underlying causal factor so to arrive at final diagnosis, it need thorough examination of animal along with sophisticated techniques.
1. Clinical History: The appropriate clinical history can rule out many causes of RBS.
2.Oviduct defects/ blockage/ adhesions can be diagnosed by PSP (Phenolsuplhonaphthelin dye test).
3. Ovulatory defects of anovulation and delayed ovulation can be ruled out be per rectal palpation of genitalia or by using transrectal ultrasonography. Palpation of ovulatory follicle at 24 h after standing estrus indicates ovulatory defect.
4. Luteal insufficiency can be ruled by estimation of plasma or serum progesterone concentration.
5. Subclinical endometritis diagnosed by uterine cytology by Cytobrush technique. The cytobrush technique is superior in all respects as more consistent and reliable method than the lavage method and accurately diagnose based on the PMNs cells % in the uterine sample collected using the cytobrush assembly. Modified Giemsa staining is done to evaluate smears. In a more than 50 days postpartum animal, if PMNs % is more than 5% then, it is termed as subclinical endometritis.


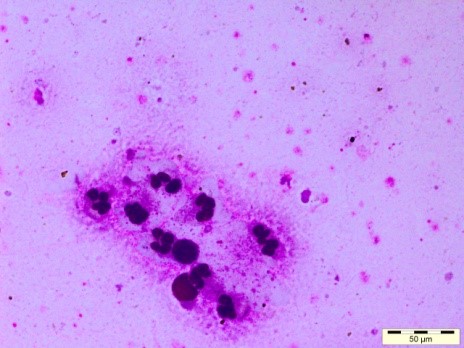
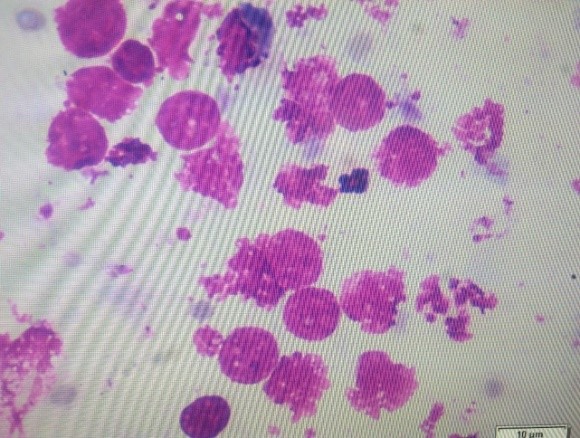
Fig. 3: Uterine Cytobrush assembly and Giemsa stained cytology smears with PMNs and uterine epithelial cells
Therapeutics/ management of RBS
- Animal should be maintained on good ration with proper housing. Rest before and after AI is advised because elevated cortisol interferes with LH secretion thus interfering with ovulation. Feeding of mineral mixture regularly @ 50-100g on daily basis should be done.
- The AI should be done in late estrus by following AM-PM rule (animal coming in estrus at morning should be inseminated in evening). At least twice insemination at 12 h interval should be followed. Bull parading is advised thrice (every 8h) a day in the herd for proper estrus detection. Semen quality for the AI must be good with more than 50% post-thaw motility, lesser sperm abnormalities. Thawing of semen should be done at 37°C for 30s and thawed semen must be used within 5-10 minutes for AI.
- Progesterone injection at time of AI never to be used as it leads to failure of fertilization.
- Animal diagnosed with subclinical endometritis should be treated with intrauterine administration of antibiotics like Cephalexin, Levofloxacin or Cephapirin (drug of choice).
- To compensate ovulatory defects injection Buserelin acetate 10µg/ Chorulon 1500IU should be administered with first AI. This shall also help in better CL development.
- To overcome luteal insufficiency either injection progesterone at day 5/12/both post-AI (500 mg hydroxy progesterone caproate) or Injection buserelin acetate at day 5/12/both post-AI (10 µg) or Injection hCG at day 5/12/both post-AI 1500 IU) can be used.
Conclusion: Good quality semen from disease free bulls should be used for AI with AM-PM rule and twice AI at 12 h interval. Subclinical endometritis should be treated by intrauterine drugs. Use of GnRH/hCG at first AI, Progesterone/GnRH/hCG at day 5 and 12 post AI compensates ovulatory defects and luteal insufficiency, respectively. If still animal fails to conceive then culling of the animal is recommended.
Amarjeet Bisla1, Nakul Gulia2*, Vinay Yadav3 and Mrigank Honparkhe4
1,3Scientist, 2Assistant Professor, 4Principal Scientist-cum-Head
Department of Veterinary Gynaecology and Obstetrics, College of Veterinary Science (Ludhiana), Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana-141004, Punjab, India
*Corresponding author: Nakul Gulia, Assistant Professor (Email: nakul@gadvasu.in)









